When I first started this blog, it seemed very easy to write about a multitude of topics that piqued my interest that weren’t related to my work. However, I feel like I’m doing a lot of heavily disclaimered blog posts lately, making it clear that this blog is a creation of my own entirely separate from my day job. Whether that’s because the things we’re looking into at work are genuinely fascinating to me or because we’re constantly identifying more things that are relevant to our organizational focus, I don’t know. It’s probably a little of Column A and a little of Column B.
All this to say that I had this blog post planned before several nonprofits in the Pittsburgh region picked up this topic of interest – I’m not out of material and mining other peoples’ work. In fact, I am writing about hydrogen as a fuel because it is 1) genuinely interesting on its own, and 2) poised to be a critical piece of our energy economy in the coming years. There are many pieces to understand – not just with the fuel itself, but the ramifications of how we make and use it – and this post will only touch the tip of the iceberg.
Hydrogen as a Fuel
When we talk about hydrogen as an alternative fuel source, we are generally touting the benefits of hydrogen as a clean-burning form of stored energy. There are two ways it can be used, which (at that point in the lifecycle – where it’s being used) represent cleaner alternatives to burning fossil fuels: hydrogen fuel cells and hydrogen combustion. There are some very specific associations with hydrogen combustion, such as the Hindenburg, but hydrogen actually is far less explosive than fossil fuels.[1]
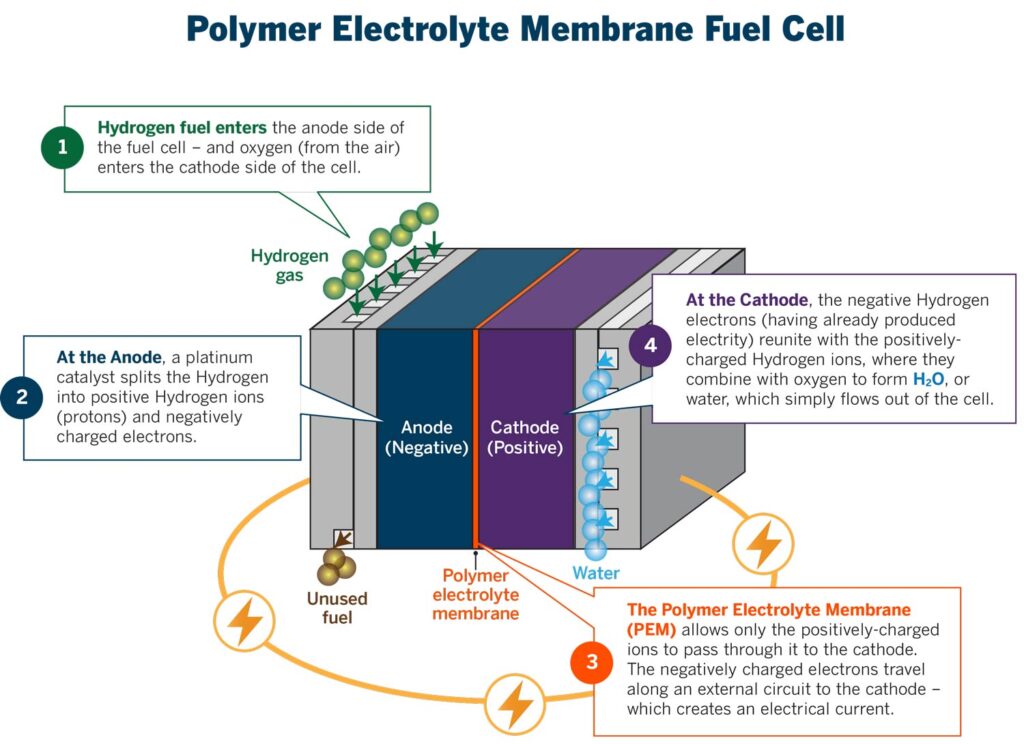
Image credit: [2]
Hydrogen combustion can be used for industrial processes that need high heat, such as concrete production.[3] Burning hydrogen in atmospheric air – where most of us live our lives – produces nitrogen oxides, which are harmful to human health. Hydrogen burns cleanly in pure oxygen, but that isn’t very feasible or cost effective on the scale of industrial applications.[4]
Hydrogen fuel cells use hydrogen and air to generate an electric current; this method produces water as a byproduct.[5] Hydrogen fuel cells can be used to power homes, cars, and pretty much anything that runs on electricity. I first heard about this technology in my sustainability tools class in grad school and remember thinking how it sounded too good to be true. And if something sounds too good to be true, it often is.
An Energy Panacea?
The thing to keep in mind with hydrogen is that at the point of use – whether it’s in a fuel cell or being burned – there are far fewer emissions than using fossil fuels for the same purpose, which is great. Decarbonizing our transportation, energy, and production sectors will be critical in addressing the climate crisis. However, the questions we need to ask – consistently – are “how was the hydrogen created?” and “what were the impacts of that process?”

Image credit: [7]
If you’ve been at all plugged into this conversation about hydrogen fuel sources, you may have heard terms like “blue hydrogen” or “green hydrogen.” The hydrogen itself is still a molecule consisting of two hydrogen atoms, still a colorless and odorless gas, still a commodity that doesn’t actually look any different in appearance. The color labels are intended to identify how the hydrogen was produced.
Still relatively new to the subject, I initially thought that the colors related to the source of energy used to produce the hydrogen through an otherwise identical process. In reality, there are multiple ways of creating hydrogen, and some are significantly more environmentally friendly than others.[8],[9] There are also many more processes and colors than I originally thought…
Water Electrolysis, a.k.a. electrochemical water splitting is “the process of using electricity to decompose water into oxygen and hydrogen gas.” Inputs to the process are water and electricity; outputs are hydrogen and oxygen. The question we must ask here is how is that electricity generated? And fortunately the three colors linked with this process are associated with carbon-free energy sources: renewables (with a specific nod to solar) and nuclear.
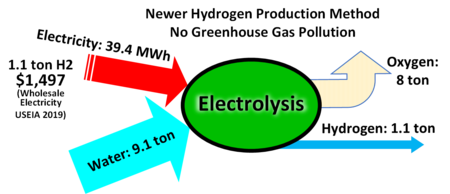
The energy value of the hydrogen (as of the writing of this post) is about 80%, meaning that of the energy that is used to create the hydrogen, about 80% of it can be utilized when the hydrogen is put to use.[11]
- Green hydrogen – water electrolysis powered by renewable electricity (wind, etc.)
- Yellow hydrogen – water electrolysis using electricity from solar power (also renewable, so it is a subset of green)
- Pink hydrogen – water electrolysis using electricity from nuclear power (zero-carbon, as there are no greenhouse gas emissions at the point of energy generation)
Steam Methane Reforming (SMR) “mixes natural gas with very hot steam, in the presence of a catalyst, where a chemical reaction creates hydrogen” and other byproducts, such as carbon monoxide and carbon dioxide. The biggest concern with this method is that it uses methane – a potent greenhouse gas – to produce the hydrogen, so while the hydrogen itself may be clean, the process of making the hydrogen is not. The two variations on this process refer to whether or not there is carbon capture technology built into the system to deal with the outputs. Blue hydrogen is being touted as environmentally friendly because of the claims that it can capture the resulting CO2 and store it underground. However, there is a lot of skepticism around whether the technology can do that effectively and whether the CO2 will stay where it is put.[12]
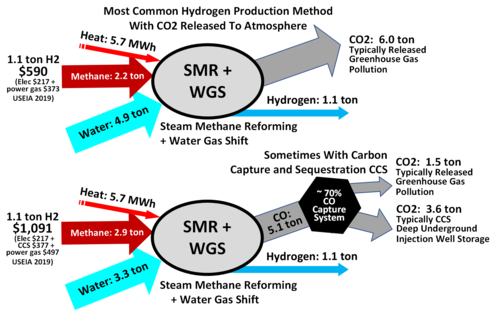
The energy value of hydrogen created through SMR is about 65%, making it less efficient a process than electrolysis, in addition to having fossil inputs and GHG outputs.[14] SMR represents the bulk of hydrogen production, at almost 3/4 of the market.
- Blue hydrogen – produced from fossil fuel, but CO2 captured and stored underground
- Gray hydrogen – produced from fossil fuel, without carbon capture
Pyrolysis is distinct from SMR because the only inputs are heat and methane. Without the inclusion of heated water/steam, the outputs of this process are hydrogen and solid carbon. Again, the concern here is that this process makes use of a fossil fuel for the creation of a cleaner, decarbonized energy source.
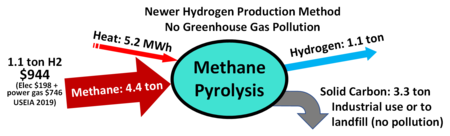
The energy value of this method is lower than the others, as well, at 35-50% efficiency.[16]
- Turquoise hydrogen – methane pyrolysis, removes carbon in solid form instead of CO2
Coal Gasification is a process used to create gas by combining coal or biomass with air, steam, and heat to produce gasses and slag. The produced gasses can then be treated further to produce hydrogen. The process itself was used historically to supply homes with gas for heating and lighting before methane extraction was as prevalent as it is today.[17] The two hydrogen colors associated with this process refer to the various fuel inputs, specifically coal and lignite.
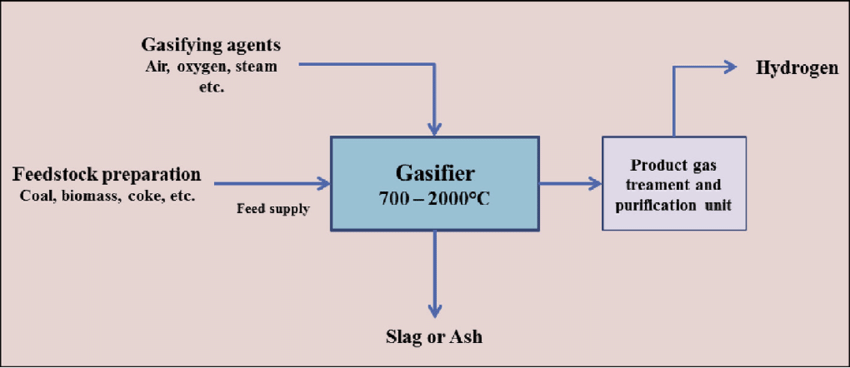
The energy utilization factor is around 65%, and it represents about a quarter of hydrogen production.[19]
- Black hydrogen – gasification of bituminous coal
- Brown hydrogen – gasification of lignite
Thermochemical water splitting, or “thermolysis,” is similar to electrolysis in that it uses water as an input, and gets hydrogen and oxygen as outputs. The difference is that where electrolysis uses electricity to do the work, thermolysis uses extremely high temperatures. Since nuclear reactors get very hot and produce electricity, these types of hydrogen production make use of that otherwise wasted heat, either by itself or in combination with electrolysis.
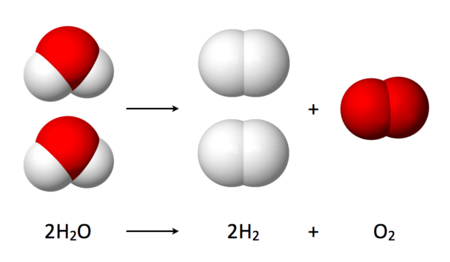
- Red hydrogen – high-temperature catalytic splitting of water, making use of waste heat from nuclear power generation
- Purple hydrogen – lesser-known and described as “nuclear power and heat through combined chemo-thermal electrolysis splitting of water,” [21] which I took to mean some combination of thermolysis and electrolysis
Naturally Occurring hydrogen refers to hydrogen in its natural state, as it exists in our world. There are no real strategies for hydrogen capture from the environment, and it is unclear if anyone is even looking into it.
- White hydrogen – “naturally occurring hydrogen in its most natural state” [22]
The vast majority of hydrogen is still produced from fossil fuel sources, as it is the most cost effective, even if it is inefficient with more environmental concerns. In 2020, 76% of the world’s hydrogen was produced from methane gas, and 23% was from coal, leaving only about 2% coming from renewables.[23] I heard at a conference this summer that the price point on renewable hydrogen is improving and should see a tipping point sometime in the next 10 years. That’s good news for the world 10 years from now, but not if we commit wholly to more fossil fuel-based hydrogen production infrastructure.
While there are arguably some processes that cannot be decarbonized without hydrogen – and the bulk of that hydrogen is produced with fossil fuels – we need to examine just how much good a hydrogen economy transition will do and whether there are intermediate steps we can be taking. This technology and policy decisions around it are developing rapidly, so I’m sure there will be updates to come in the future.
I’m curious to hear your thoughts below about what we should or shouldn’t be using and why.
Thanks for reading!
[1] https://www.nrdc.org/experts/christian-tae/hydrogen-safety-lets-clear-air
[2] https://www.sundyne.com/how-does-a-hydrogen-fuel-cell-work/
[3] https://radicalmoderate.online/concrete/
[4] https://pubs.rsc.org/en/content/articlepdf/2021/ea/d1ea00037c
[5] https://www.energy.gov/eere/fuelcells/fuel-cells
[6] https://wot.fandom.com/wiki/Ajah
[7] https://www.artstation.com/artwork/wlOZV
[8] https://www.h2bulletin.com/knowledge/hydrogen-colours-codes/
[9] https://www.nationalgrid.com/stories/energy-explained/hydrogen-colour-spectrum
[10] https://en.wikipedia.org/wiki/Electrolysis_of_water
[12] https://onlinelibrary.wiley.com/doi/10.1002/ese3.956
[13] https://en.wikipedia.org/wiki/Steam_reforming
[15] https://en.wikipedia.org/wiki/Pyrolysis
[16] https://link.springer.com/referenceworkentry/10.1007/978-1-4939-2493-6_956-1
[17] https://en.wikipedia.org/wiki/Coal_gasification
[19] https://www.sciencedirect.com/science/article/abs/pii/S0360544212002575
[20] https://en.wikipedia.org/wiki/Water_splitting
[21] https://hydrogeneurope.eu/in-a-nutshell/
[22] https://www.nationalgrid.com/stories/energy-explained/hydrogen-colour-spectrum
[23] https://home.kpmg/xx/en/home/insights/2020/11/the-hydrogen-trajectory.html
0 Comments